In 2024, SciBase continued developing its commercialization strategy and achieved strong growth. With a new U.S. organization on site and a broadened customer base, sales increased by 222%. Nevisense gained further validation in the U.S. with the publication of a consensus report confirming the value of Nevisense's AI-based technology in early detection of melanoma. Germany continues to deliver profitable sales growth, and the Company expanded its geographic reach with new distributor agreements in Italy and the United Arab Emirates. The launch of the AI-powered eBarrier Score, along with the publication of several important clinical studies within skin barrier assessment, have sparked interest among both researchers and the cosmetic industry. SciBase began collaborations with key institutions and organizations, including Skinobs, Seraly Dermatology, SKIN Research Group, and, after year-end, Mayo Clinic. To sustain its accelerated growth, SciBase prioritizes three strategic areas: continued expansion into the U.S. through broader reimbursement, continued profitable sales growth in Germany while addressing new European markets, and advancing applications in skin barrier assessment.
Continued growth in Germany, our core market
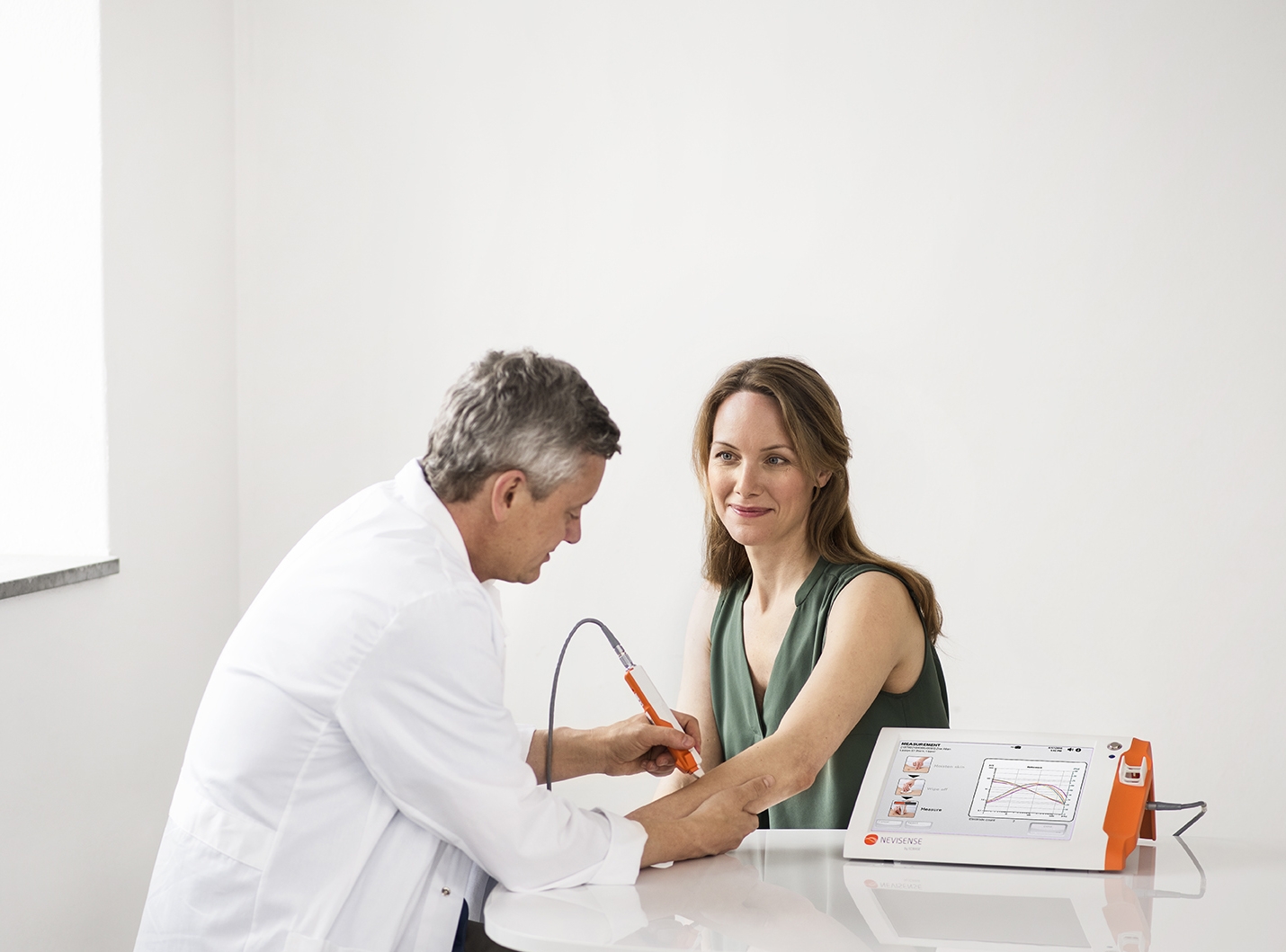
- SciBase is experiencing continued profitable sales growth in Germany, our core European market
- Currently, over 450 Nevisense systems are installed at around 370 private dermatological clinics
- With over 260,000 patients clinically tested for skin cancer, we're making a significant impact on early detection
- In 2024, sales increased by 10% compared to the previous year
- We proudly hold MDR certification in Europe, a significant barrier to potential competitors.
- Our profitability and robust sales growth in Germany demonstrate our market leadership.

Strategic partnership with Kenvue (formerly J&J Consumer Health)
- Our partnership with Kenuve is a testament to our potential
- Collaborating with Swiss hospitals, we're developing a screening product to predict the onset of atopic dermatitis (AD) in infants.
- Significant unmet needs exist in skin barrier-related conditions like eczema and food allergies, with 20% of infants developing AD.
- Notably, key articles published in 2021, 2022 and 2023 generated significant interest in our skin barrier products.
- Nevisense and Nevisense Go have been selected for several large studies, paving the way for future growth.
- Nevisense Go, our handheld platform, targets the barrier research market initially and will expand to dermatologists, non-specialists, and home users.
- We're actively partnering with industry leaders to support AD therapy and management through our Nevisense platforms.
Unlocking US market reimbursement for continued growth
- SciBase's Nevisense is the sole FDA approved point-of-care device available for melanoma detection in the US.
- Our success is validated by the enthusiastic adoption of our technology at the first 60 US sites.
- Substantial progress has been made in securing Medicare reimbursement, with the first two regions covering our method through a fee schedule.
- We are actively engaged with private payers and additional Medicare regions to expand accessibility.
- Our presence in the US market holds immense growth potential.
- Collaborations with large dermatology practice groups, such as ADCS, and clinics focused on skincare and special services are driving cost-effective market penetration.

